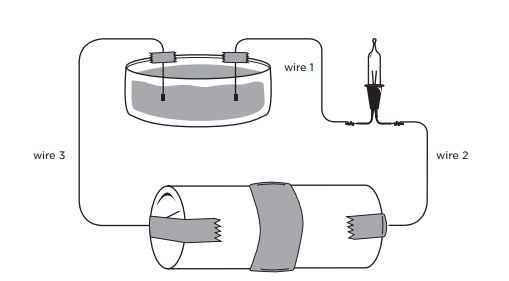
A circuit is a continuous path through which electricity travels. We usually think of this path as a wire, but believe it or not, it can also be a liquid!
To conduct electricity a substance needs charged particles that are free to move.
It might surprise you to hear that water is a poor conductor, since you've been told never to use an electrical appliance near water because of the danger of electrocution.
Absolutely pure water wouldn't let charge move through it, although it's particles are always moving, they are not charged, so it wouldn't conduct poor conductor of electric current. Everyday water around our houses or in nature always has other substances dissolved in it, and those dissolved substances may allow charge to move.
Salt is poor conductor, as its solid molecules are locked in place and cannot move.
Salt molecules are made of charged sodium ions and chlorine ions. When you put salt in water, the salt dissolved into solution. Water molecules pull the sodium and chlorine ions apart so they are floating freely. These ions are what carry electricity through water, as these charged particles can freely move.
In this demonstration, students will see how a solution can complete a circuit.
Fun Fact!
A substance may be an insulator if the voltage is low, but may conduct current if the voltage is higher. The mini-light in this circuit won't glow if there's a gap between the wires – air doesn't conduct current at this low voltage. With the higher voltages in a thunderstorm, the air molecules are ripped apart into ions and will conduct current quite well – that's why you see lightning!

