Objectives
-
Describe the movement of electrons from one material to another.
-
Determine the resulting charge of two materials rubbing together.
-
Explain how static charge causes materials to attract or repel each other.
Materials
Background
Everything we see is made up of tiny particles of matter called atoms. The atoms are made up of even smaller parts called protons, electrons and neutrons. An atom usually has the same number of protons and electrons, but sometimes electrons can be moved away from their atoms.
If you comb your hair, for example, electrons leave the atoms and molecules in your hair and travel to the plastic comb. The comb, covered in negatively charged electrons, becomes negatively charged as well, and your hair is left with a positive charge. This “separation of charge” is the reason for the collection of effects we call static electricity.
If two objects have different charges, they attract (or pull towards) each other. If two objects have the same charge, they repel (or push away) from each other. After you’ve combed your hair, every single hair has the same positive charge. Since things with the same charge repel each other, the hairs try to move away from each other by standing up and away from all the other hairs, resulting in you having a very funny-looking hairdo!
Another example: if you walk across a carpet, electrons move from the rug to you. Now you have extra electrons. If you have extra electrons piled on you, they will spill off when you touch an object like a doorknob, and give you a shock. Shocks come from gaining or losing electric charge in a hurry.
When a charged object is brought close to a neutral material, the electrons on the neutral material will either move toward the charged object (if it has a positive charge) or away from the charged object (if it has a negative charge). In other words, the neutral material “picks up” charge on its near and far side, relevant to the charged object. This phenomenon is called an induced charge. The result is that a normally neutral material will have a slight charge when near the charged object, and it is enough for the two to attract.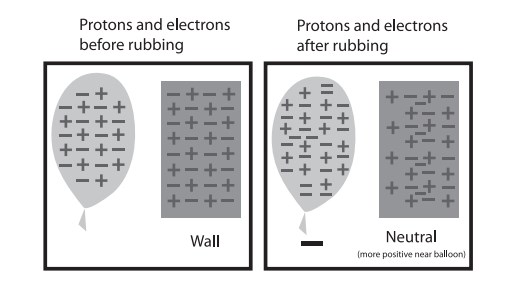
Electrostatic charges are not caused by friction, although many assume this to be the case.
Rubbing a balloon on your head or dragging your feet on the carpet will build up a charge, but so will ordinary walking or repeatedly touching your head with a balloon! It’s the mere contact between two different materials that causes charge to move from one object to another. Rubbing materials together can help move charge more quickly because more surface area is being contacted. Friction has nothing to do with the charge.
An important thing to consider when doing any of these activities is the weather: humidity in the air can make it difficult to build up charges, causing experiments to behave in unexpected ways!
The best “static” weather is clear, sunny, and cool.
Vocabulary
atom – the particle of matter made of protons, electron and neutrons
electron – A subatomic particle that has a negative electrical charge.
electroscope – A device that detects electrical charge.
induced charge – Separation of charges within an otherwise neutral object caused by the proximity of a charged object.
proton – A subatomic particle that has a positive electrical charge.
static electricity – Electrical effects caused by the charge imbalance between a negatively charged object and a positively charged object.
Triboelectric series – A list that ranks various materials according to their tendency to gain or lose electrons.
Other Resources
BC Hydro | Power Smart for Schools
How Stuff Works | How do Van de Graaff Generators Work
To purchase a fly stick or Van de Graaff generator: Arbor Scientific


