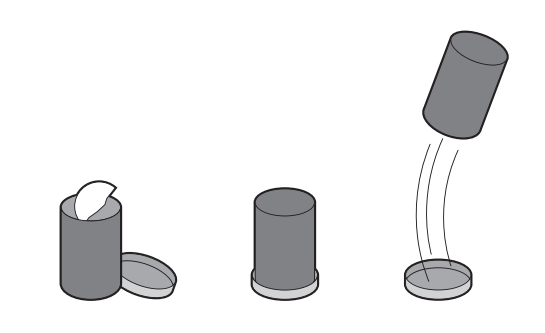
In this activity, students construct a rocket using a canister with a snap on lid and set it off with an effervescing antacid tablet.
Alka-Seltzer contains citric acid and sodium bicarbonate (baking soda). When you drop the tablet in water, the acid and the baking soda react to produce carbon dioxide gas.
The CO2 gas pushes against the lid of the canister until there is so much pressure that the lid pops off. When the gas explodes downwards, it pushes the rocket in the opposite direction, causing it to go up (Newton’s Law of Motion).
This system of thrust is how a real rocket works, whether it is in outer space or here in the earth's atmosphere. The difference is that real rockets use rocket fuel.
This activity is best done outside or in a large space (like a gym) that is easy to clean.