We all know states of matter can be solids, liquids or gases. But what about substances that are gooey? In our Super Science Club (SSC), kids have a great opportunity to learn some fun chemistry. We begin our session with a magic trick…except it’s not magic, its science!
Have you ever heard of sodium polyacrylate? I know I hadn’t until Super Science Club, but it turns out this substance is pretty useful and it’s in a lot of everyday products you might use at home. Let’s think about diapers for a moment, why don’t they leak every time a baby has a wee? Because it contains a chemical called sodium polyacrylate that absorbs all the liquid! Can you think of anything else it might be used in?
Many modern products we use today are polymers. Poly, meaning many, and mer meaning parts. Sodium polyacrylate is a polymer, so we show the kids exactly how this works by mixing some of the powdery sodium polyacrylate (slush powder) with water. We start off the demonstration with three “empty” cups. What the students don’t know is that in one of the empty cups we have sneakily poured in some slush powder. We then pour some water into one of the cups, and ask the kids to try to remember which cup has the water as we perform something similar to the three shell game many magicians perform.
The cool thing about sodium polyacrylate powder is that it can absorb up to 800 times its own weight and it sticks to the bottom of the cup! Then we set up our discrepant event. We get three volunteers and we pour each cup over their heads. Unexpectedly, no one gets wet, which opens the floor for some scientific inquiry. Where did the water go? What are some possible explanations? We then pass this polymer around for all the kids to observe and touch.
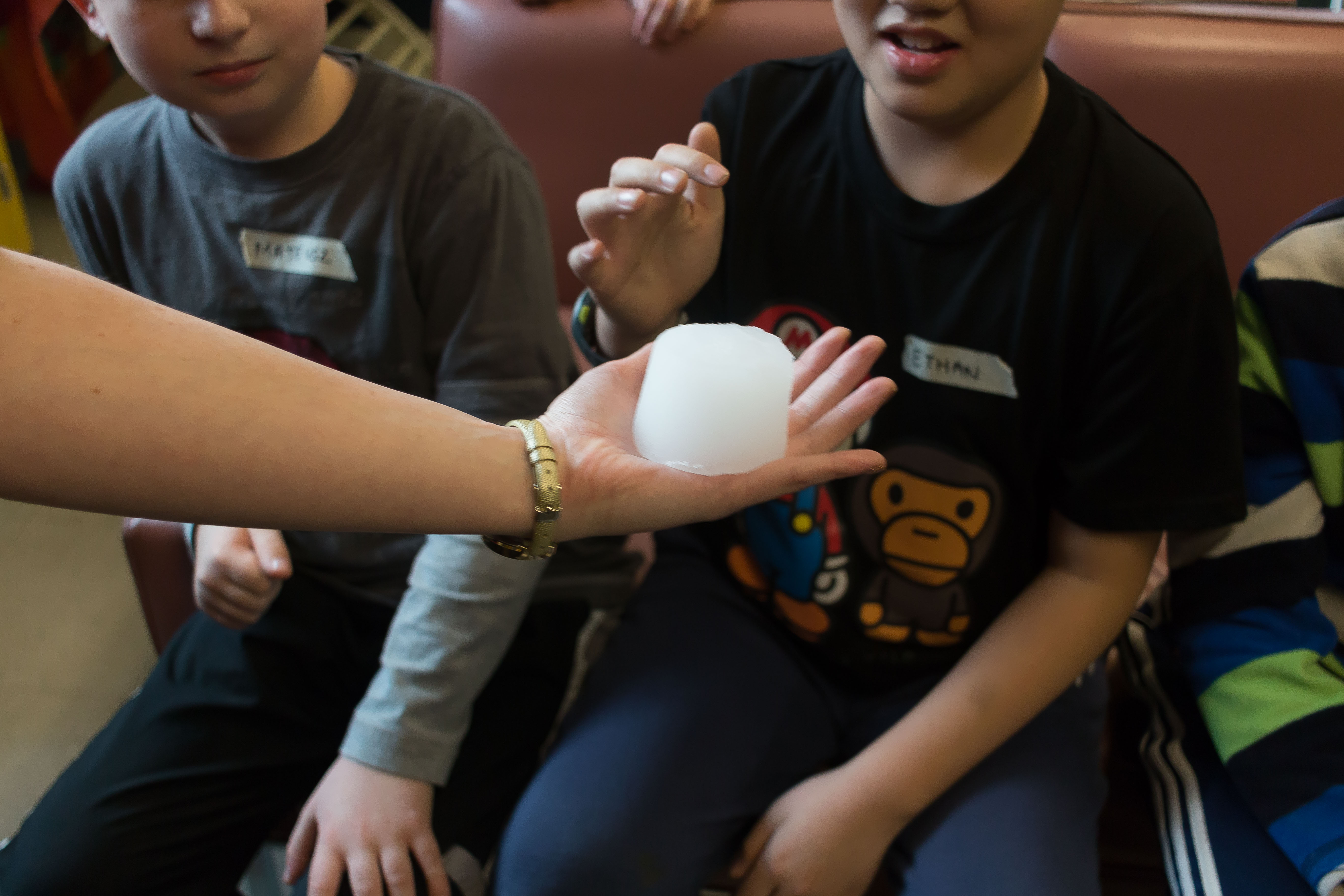
As you can see in the picture above, the material is not quite a solid, a liquid or a gas, but instead is something rather squishy. “I just want to touch this forever!” was a great quote from one of the kids at James Cook School. It certainly has a unique texture! After our demonstration we discuss why and how this chemical reacted the way it did and what it’s many uses could be.
For every lesson we also do a super fun activity where the kids make something they get to take home. In this lesson about goo and polymers, the kids make something very delicious…homemade gummy worms! Check out our free resources page to see how you can make your own yummy gummies at home.
We have a lot of fun in our goo lessons and the kids are always so curious. “If a polymer means many parts, what would one part be?” Great question! The answer is a monomer, mono meaning one! They always challenge us as facilitators and we learn a ton while having an awesome time. Goo is entertaining, silly, delicious, squishy and very useful!
Sodium polyacrylate is a chemical and in the demonstration mentioned above, it definitely underwent a reaction. But what do you think? Was it a physical or a chemical reaction? Let us know in the comments below.